In Gene Therapy, Data is as Critical as Biology
QuTEM is a Core Investment of Katalysen; a strategic position in the industrialization of quality control for next-generation gene therapies.
Market Shift: Gene therapy is moving from science to industrial scale
Gene therapies are transitioning from experimental trials to large-scale commercialization. As more therapies reach approval, global manufacturing demand is expected to multiply dramatically — with quality assurance emerging as one of the most critical bottlenecks to safe deployment.
Strategic Positioning: The control layer before regulators and manufacturers will allow scale
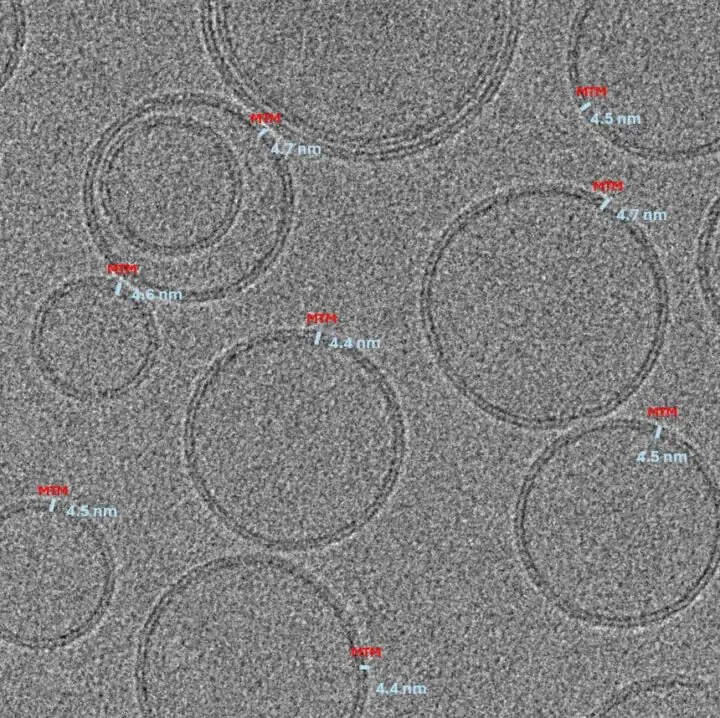
Regulatory approval and industrial adoption now hinge on proving that every dose is safe, consistent, and precisely understood at a sub-nanometer level. QuTEM sits exactly where quality becomes binary — the data layer that determines whether a therapy can be approved, released, or reimbursed.
Solution Edge: GMP-grade electron microscopy analytics with proprietary software and data
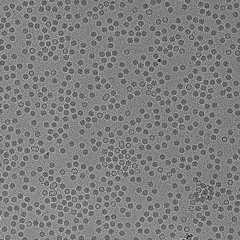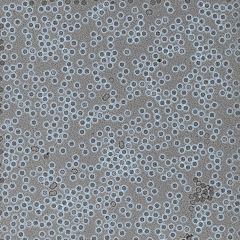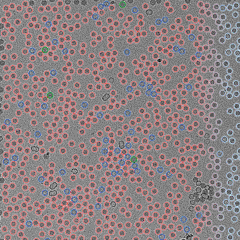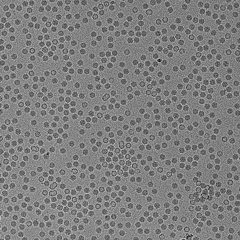
QuTEM combines high-precision electron microscopy with a software-driven analytics engine (Gridsee), built over years of GMP-certified work. This enables far higher fidelity than subjective manual inspection — producing standardized, auditable data that can be directly integrated into regulatory, QA, and manufacturing automation systems.
Team & Moat Logic: Deep scientific and regulatory credibility, built inside real GMP environments
The founding team brings rare intersection experience across electron microscopy, GMP manufacturing, and regulatory processes — not theoretical, but battle-tested inside live production environments. QuTEM is not a speculative tool — it is already trusted in contexts where no mistakes are tolerated.
Traction & Forward Motion: Already integrated into live industrial production, expanding capacity now
QuTEM is already deployed commercially with some of the world’s leading pharmaceutical companies, with growing demand from global gene therapy leaders preparing for large-scale approvals. The company is now scaling infrastructure to meet industrial-level demand as manufacturing volumes accelerate globally.