In Diagnostics, the Next Phase Begins Before the Lab
S4DX is a Core Investment of Katalysen; our position on the accelerating global surge in blood diagnostics — digitizing the pre-analytics layer where errors originate and value is created first.
Market Shift: Blood sampling is exploding, but still relies on manual, analog procedures
Global demand for blood testing is rising sharply due to aging populations, personalized medicine, and expanding chronic disease monitoring. Yet the pre-analytics step — blood collection and handling — remains largely manual and prone to human error, far behind the level of automation seen inside modern laboratories.
Strategic Positioning: Controlling the part of diagnostics still untouched by digital standardization
Laboratories have already modernized — but the sample that arrives is only as good as the conditions under which it was collected and transported. S4DX is positioned at the exact point where preventable errors begin — before they enter the lab — enabling traceability, standardization, and real-time data where none existed before.
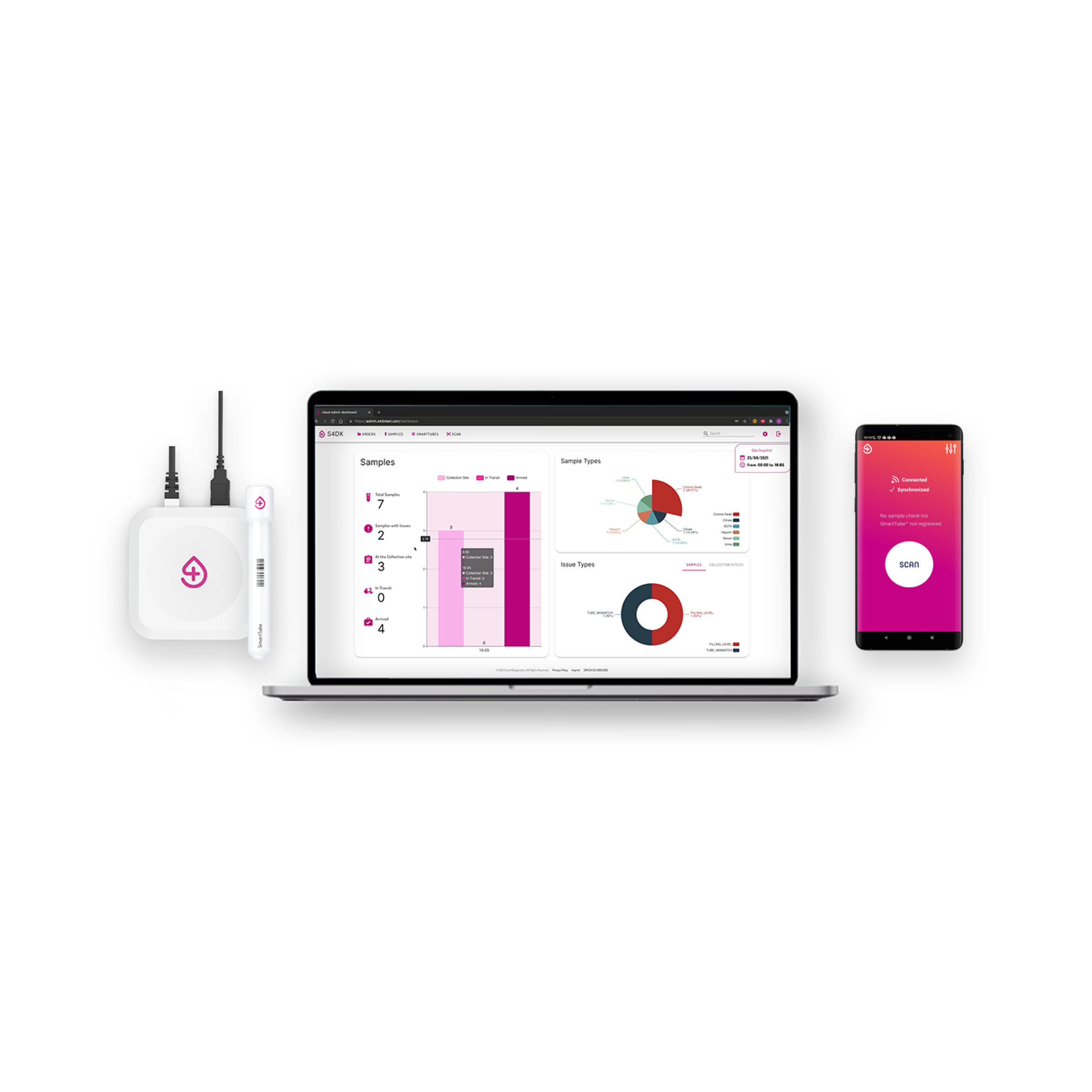
Solution Edge: A software-driven platform that digitizes and validates every step of sample collection
S4DX replaces analog handling with a fully traceable, data-native pre-analytics infrastructure — capturing temperature, movement, time, and custody events per sample. This enables hospitals and laboratories to eliminate uncertainty, improve quality assurance, and meet rising regulatory requirements for auditability and automation.
Team & Moat Logic: Built by experts from biomedical engineering, diagnostics, and regulated automation
The S4DX team unites medical instrumentation experience with deep knowledge of clinical workflows and regulatory production logic. Their platform is not theoretical — it is designed to integrate into highly regulated environments where compliance, reliability, and data integrity are non-negotiable.
Traction & Forward Motion: Working with the largest IVD providers across Europe, including first UK hospital deployment
S4DX is actively deploying its platform with major in-vitro diagnostics providers across Europe — and has recently also entered the UK market with its first first hospital implementation in the region. The company is now positioned to scale adoption as regulators push healthcare systems to eliminate analog uncertainty from their diagnostics workflows.